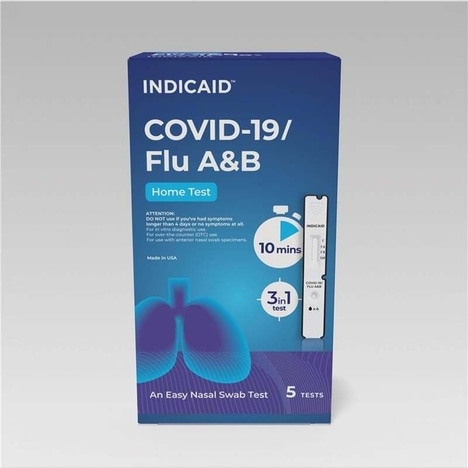
The test kit only requires one nasal swab to be performed, which can then be used to test for COVID-19, Flu A and Flu B with results in just 10-minutes. The test kit is FDA 510(k)-cleared to ensure it’s backed by validated clinical performance, is made in the US and cleared for over-the-counter sale. The kit will start hitting store shelves starting August 11, 2025.
Founder and CEO of PHASE Scientific Ricky Chiu spoke on the INDICAID COVID-19/Flu A&B Home Test saying, “This product reflects our belief that diagnostics should be accessible, accurate and actionable. The Indicaid [COVID-19/Flu A&B] 3-in-1 [home test] is a trusted solution for everyday health decisions, made available in the United States and cleared through the FDA’s rigorous review.”









